The history of acrylic rubber goes back to the synthesis of acrylic acid and methacrylate acid in the middle of the 19th century. But the manufacturing potential associated with acrylics was revealed around 1901 when the German chemist Otto Rohm (1876-1939) published his research on acrylic esters. Later, around 1930, the synthesis of compounds related to acrylics, including acrylic elastomers, flourished. Today, many rubber products are produced using ACMs, which we will further examine these rubbers.
ACM:
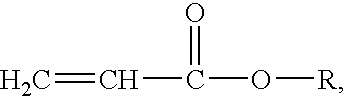
Polyacrylic elastomers, also called ACM rubbers, are synthetic elastomers synthesized from acrylic monomers. These polymers are usually prepared by emulsion polymerization. The main repeating units are usually ethyl, butyl acrylate or a combination of the two.
Chemical structure:
Properties of ACM rubber:
This rubber has good resistance to ozone and weathering, which makes them superior to nitrile rubber (NBR). But it does not have good resistance against water, humidity, acids and bases. In addition, due to its relatively low flexibility at low temperatures, it is not suitable for applications below -10 degrees Celsius.

Application of ACM rubber:
ACM rubber is mainly used in cases where combined resistance to heat and oil is required. This rubber is used in internal car components such as hoses that need to withstand hot oil, fuel and many other common car lubricants and hydraulic fluids. Also, due to their good resilience, these tires are used in industry for applications that require vibration damping. The application temperature range of this rubber is continuously from -10 to 150 degrees Celsius, which can be used for a limited period of time for more than 150 degrees. These rubbers are an excellent alternative to more expensive heat-resistant elastomers such as fluorocarbon polymers (FKM), vinyl methyl silicone (VMQ) and fluorosilicones (FVMQ) for high temperature applications (more than 150 degrees Celsius) are
Types of ACM:
Acrylic rubbers have two grades:
- Soft grade (A60-A90)
- Film grade






