Ethylene-propylene rubber was first made available in small amounts in the United States in 1962. Small quantities were made in Italian and American labs between 1954 and 1960. Although commercial manufacturing of ethylene-propylene rubber only started in 1963, it currently has the highest growth rate.
History of EPDM:
The history of this rubber is intertwined with the production of ethylene and propylene polymers. In 1951, German scientist Karl Ziegler (1898-1973) invented an entirely new class of catalysts for polymerization reactions. These catalysts were composed of a mixture of transition metal halogens and an organic-metallic reducing agent, such as alkyl aluminum. Low-pressure production of linear high-density polyethylene represented the first commercial application of Ziegler catalysts.
In Italy, Professor Giulio Natta (1903-1979) expanded research on this category of catalysts and demonstrated that some of them are capable of producing numerous polymers that typically differ from other types in terms of their spatial arrangement. Consequently, these catalysts are known as stereo-specific catalysts. Nata also discovered that propylene could be polymerized in this manner, resulting in the marketing of polypropylene as the second olefin plastic.
Polyethylene and polypropylene are thermoplastics that lack any rubber-like properties. Nata discovered that ethylene and propylene can be co-polymerized in an irregular and random manner using certain types of Ziegler catalysts to produce an amorphous material with excellent elasticity and rubber properties. Ziegler and Nata were awarded the Nobel Prize in Chemistry in 1963 for their discovery that led to the development of a number of novel elastomers and plastics, including ethylene and propylene rubbers.

EPDM:
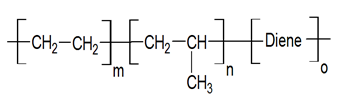
Ethylene, Propylene, Diene is a monomer that provides cross-linking sites for vulcanization. It is a copolymer of ethylene, propylene, and a minor amount of unconjugated Diene monomers (between 3 and 9 percent). This is known as cross-linking.
DN can typically take on these three forms:
- Ethylidene Nobornene (ENB)
- Dicyclopentadiene (DCPD)
- 1,4-Hexadiene (1,4-HD)
Chemical structure:
EPDM properties:
EPDM elastomers are resistant to heat, ozone, weathering, and aging. In addition, these rubbers are excellent electrical insulators and chemically resistant. The most water-resistant rubber is EPDM. These tires have poor flammability.
EPDM applications:
Automotive is the greatest market for EPDM, which is used to manufacture radiator and heater hoses, door and window seals, o-rings and washers, accumulator bladders, wire and cable connections, insulators, and diaphragms. The combination of EPDM and other polymers (such as PP) is utilized for bumpers and fenders on automobiles. This rubber has a temperature range of -45 to 150 °C and can withstand steam temperatures of up to 180 °C.
EPDM grades:
The optimal grade of this tire is determined by the following characteristics:
- Ethylene percentage: Typical ethylene percentages in EPDM chains range from 40 to 70 percent by weight. As the percentage of ethylene increases, so does the mechanical strength of the grade. On the other hand, the hardness of the sample and its glass transition temperature also increase, but this decreases the EPDM softness performance.
- Molecular weight: as molecular weight and its equivalent Mooney viscosity increase, so does its mechanical strength. Additionally, the compression set will be reduced in this instance.
- The percentage of DN monomer: Increasing the weight percentage of DN monomer in the structure of EPDM increases the curing speed of the final compound, but decreases its compressive limit.









