Introduction: The Role of UV Light in Polymer Degradation
Synthetic polymers, when exposed to sunlight—particularly ultraviolet (UV) radiation—undergo chemical changes. The absorption of UV energy by sensitive groups (chromophores) creates excited states, leading to the formation of free radicals. These radicals trigger chain reactions of degradation, resulting in chain scission, reduced molecular weight, yellowing, and embrittlement. For this reason, nearly all plastics require UV stabilizers for outdoor use (Yousif & Haddad, 2013; Heskins, 1968).
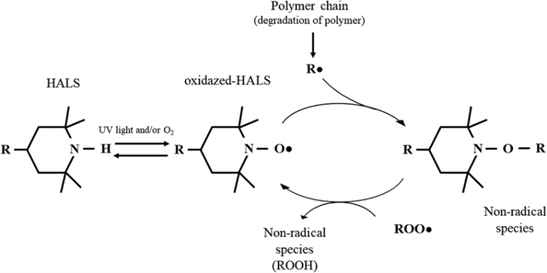
Figure 1: Mechanism of inhibition of photooxidative degradation of polymers by amine light stabilizers (HALS)
Step 1 – UV Absorbers & Light Screeners: The First Line of Defense
UV absorbers absorb UV light in the 280–400 nm range and convert the energy into heat. Common examples include benzotriazoles (C₆H₅N₃), benzophenones (C₁₃H₁₀O), and triazines (C₃H₃N₃). These are widely used in transparent plastics such as packaging films and polycarbonates, since they can be incorporated without altering the material’s color.
In addition, light screeners such as titanium dioxide (TiO₂) and zinc oxide (ZnO) reflect or scatter UV radiation, preventing its penetration into the polymer bulk. Studies (Brostow et al., 2020; El-Hiti et al., 2021) show that ZnO nanoparticles significantly reduce cracking and improve mechanical durability in polypropylene films used outdoors.
Step 2 – Excited-State Quenchers: Neutralizing Energy Before Damage
When polymers or additives absorb UV energy, molecules reach excited singlet or triplet states. If not quenched, these states can initiate degradation reactions. Quenchers dissipate this excess energy as harmless heat or non-damaging radiation.
For instance, nickel complexes are used in agricultural films to prolong the service life of polyethylene greenhouse covers. They are especially effective in thin films, preventing localized energy build-up (Karimi et al., 2020; El-Hiti et al., 2021).
Step 3 – Peroxide Decomposers: Controlling Degradation Intermediates
During photooxidation, hydroperoxides (ROOH) form as unstable intermediates. These quickly decompose into peroxy (ROO·) and alkoxy (RO·) radicals, accelerating degradation. Peroxide decomposers stabilize the process by breaking down hydroperoxides into non-radical products, preventing chain propagation.
Adding peroxide decomposers to polyethylene reduces yellowing and significantly enhances mechanical strength under outdoor conditions (Gijsman, 2017; El-Hiti et al., 2021).
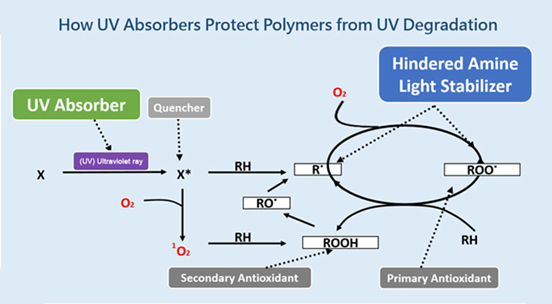
Figure 2: Mechanism of protection of polymers against UV degradation.
Step 4 – HALS: Radical Scavengers & Long-Term Protection
Hindered Amine Light Stabilizers (HALS) are the most effective UV stabilizers. They neutralize free radicals and regenerate through the Denisov cycle, maintaining long-lasting activity. Research (Gijsman, 2017) shows HALS outperform other stabilizers in polyolefins. Moreover, combining HALS with ZnO nanoparticles multiplies the outdoor durability of polypropylene (Brostow et al., 2020).
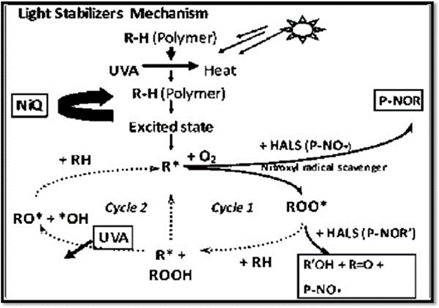
Figure 3: Mechanism of action of photostabilizers in inhibiting free radicals and preventing photooxidative degradation of polymers
Step 5 – Synergistic Effects & Choosing the Right Stabilizer
Studies confirm that combining stabilizers achieves superior protection compared to using them individually. For example, combining UV absorbers with HALS both reduces UV penetration and neutralizes radicals. Such systems are widely applied in automotive parts, outdoor equipment, and agricultural films.
The choice of stabilizer depends on polymer type, thickness, environmental conditions (humidity, radiation intensity), and cost. Even the best stabilizer may fail if improperly selected (El-Hiti et al., 2021; Heskins, 1968).
Conclusion
UV stabilizers protect plastics through multiple mechanisms:
- Absorbing or scattering UV light (UV absorbers, screeners)
- Quenching excited states
- Decomposing hydroperoxides
- Scavenging free radicals (HALS)
The combined application of these approaches provides the best results, extending the service life of plastics in outdoor applications by several times.











